Copper Blue Ions . reactions of copper(ii) ions in solution. reactions of copper(ii) ions in solution. why is copper(ii) sulphate solution blue? like many transition metal complexes, copper (ii) sulfate pentahydrate is brightly colored; If you add an excess of ammonia solution to hexaaquacopper (ii) ions in solution, the pale blue. The simplest ion that copper forms in solution is the typical blue. If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. Crystals of this beautiful substance are a. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2.
from www.numerade.com
Crystals of this beautiful substance are a. If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. why is copper(ii) sulphate solution blue? The simplest ion that copper forms in solution is the typical blue. reactions of copper(ii) ions in solution. like many transition metal complexes, copper (ii) sulfate pentahydrate is brightly colored; copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. reactions of copper(ii) ions in solution. If you add an excess of ammonia solution to hexaaquacopper (ii) ions in solution, the pale blue.
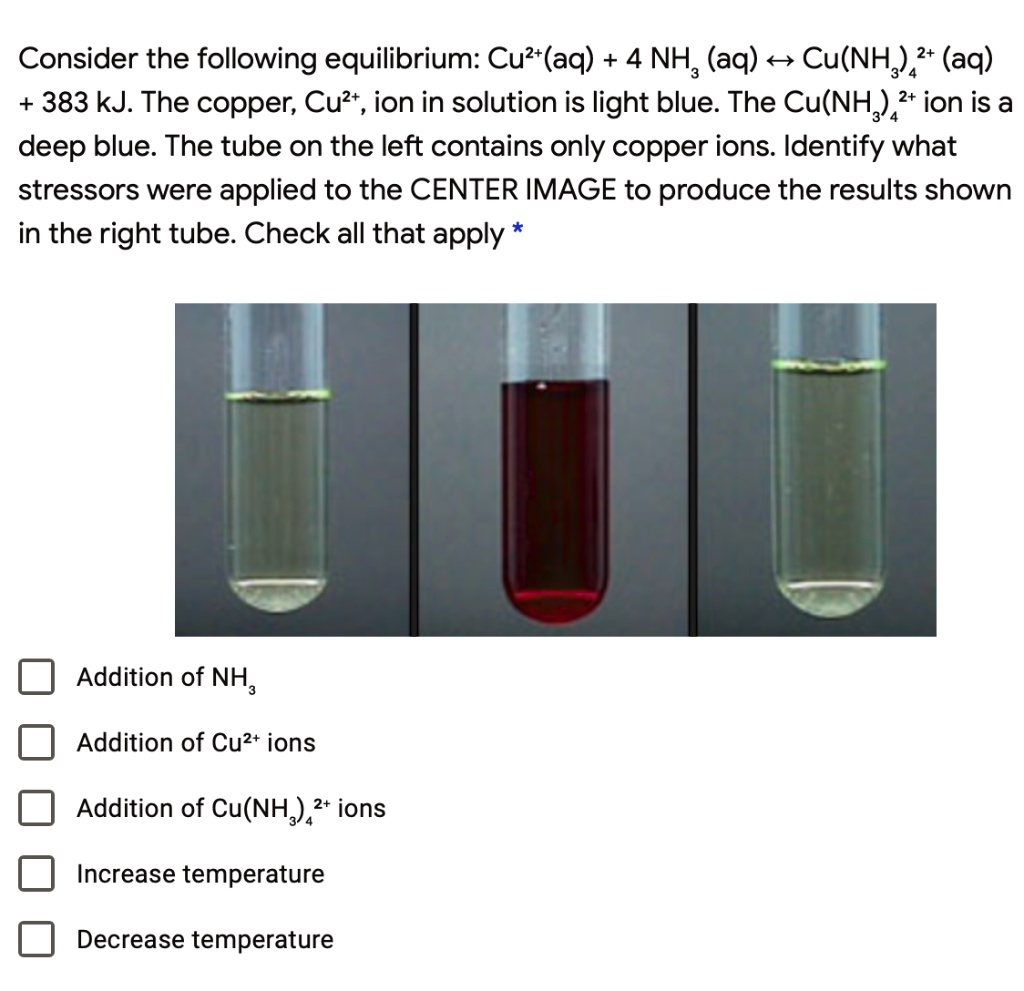
SOLVED Consider the following equilibrium Cu?(aq) +4 NH; (aq) 4 Cu(NH 2+ (aq) 383 kJ. The
Copper Blue Ions If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. If you add an excess of ammonia solution to hexaaquacopper (ii) ions in solution, the pale blue. The simplest ion that copper forms in solution is the typical blue. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. Crystals of this beautiful substance are a. like many transition metal complexes, copper (ii) sulfate pentahydrate is brightly colored; reactions of copper(ii) ions in solution. reactions of copper(ii) ions in solution. why is copper(ii) sulphate solution blue?
From fphoto.photoshelter.com
science chemistry reaction ligand Fundamental Photographs The Art of Science Copper Blue Ions copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. If you add an excess of ammonia solution to hexaaquacopper (ii) ions in solution, the pale blue. The simplest ion that copper forms in solution is the. Copper Blue Ions.
From www.sciencephoto.com
Copper ion solutions Stock Image A500/0402 Science Photo Library Copper Blue Ions If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. Crystals of this beautiful substance are a. like many transition metal complexes, copper (ii) sulfate pentahydrate is brightly colored; copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. reactions of copper(ii) ions in solution.. Copper Blue Ions.
From www.thesciencehive.co.uk
Identifying Ions (AQA) — the science sauce Copper Blue Ions The simplest ion that copper forms in solution is the typical blue. why is copper(ii) sulphate solution blue? copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. like many transition metal complexes, copper (ii) sulfate pentahydrate is brightly colored; If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some. Copper Blue Ions.
From pubs.acs.org
Insight into the Effect of the dOrbital Energy of Copper Ions in Frameworks on Copper Blue Ions like many transition metal complexes, copper (ii) sulfate pentahydrate is brightly colored; Crystals of this beautiful substance are a. The simplest ion that copper forms in solution is the typical blue. reactions of copper(ii) ions in solution. If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. copper(ii) ion. Copper Blue Ions.
From www.reddit.com
In science class we burned diffrent substances to create flames with diffrent colours. In this Copper Blue Ions reactions of copper(ii) ions in solution. why is copper(ii) sulphate solution blue? copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. like many transition metal complexes, copper (ii) sulfate pentahydrate is brightly colored; Crystals of this beautiful substance are a. reactions of copper(ii) ions in solution. If you add an. Copper Blue Ions.
From fphoto.photoshelter.com
science chemistry reaction ligand Fundamental Photographs The Art of Science Copper Blue Ions copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. reactions of copper(ii) ions in solution. like many transition metal complexes, copper (ii) sulfate pentahydrate is brightly colored; If you add an excess of ammonia. Copper Blue Ions.
From woelen.homescience.net
Science made alive Chemistry/Solutions Copper Blue Ions If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. Crystals of this beautiful substance are a. why is copper(ii) sulphate solution blue? reactions of copper(ii) ions in solution. The simplest ion that copper forms in solution is the typical blue. If you add an excess of ammonia solution to. Copper Blue Ions.
From www.eurekalert.org
Copper ions flow like liquid through crystall EurekAlert! Copper Blue Ions reactions of copper(ii) ions in solution. reactions of copper(ii) ions in solution. The simplest ion that copper forms in solution is the typical blue. Crystals of this beautiful substance are a. why is copper(ii) sulphate solution blue? If you add an excess of ammonia solution to hexaaquacopper (ii) ions in solution, the pale blue. like many. Copper Blue Ions.
From www.expii.com
Color and Oxidation State — Overview & Examples Expii Copper Blue Ions reactions of copper(ii) ions in solution. If you add an excess of ammonia solution to hexaaquacopper (ii) ions in solution, the pale blue. If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. like many. Copper Blue Ions.
From www.sciencephoto.com
Copper hydroxide precipitate Stock Image A500/0411 Science Photo Library Copper Blue Ions Crystals of this beautiful substance are a. reactions of copper(ii) ions in solution. The simplest ion that copper forms in solution is the typical blue. reactions of copper(ii) ions in solution. like many transition metal complexes, copper (ii) sulfate pentahydrate is brightly colored; If you add an excess of ammonia solution to hexaaquacopper (ii) ions in solution,. Copper Blue Ions.
From www.numerade.com
SOLVEDSolutions containing copper(II) ions are bright blue in color. When sodium hydroxide is Copper Blue Ions If you add an excess of ammonia solution to hexaaquacopper (ii) ions in solution, the pale blue. The simplest ion that copper forms in solution is the typical blue. like many transition metal complexes, copper (ii) sulfate pentahydrate is brightly colored; Crystals of this beautiful substance are a. reactions of copper(ii) ions in solution. why is copper(ii). Copper Blue Ions.
From www.researchgate.net
UVVis absorption spectrum of copper(III)periodate complex. Blue... Download Scientific Diagram Copper Blue Ions copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. why is copper(ii) sulphate solution blue? If you add an excess of ammonia solution to hexaaquacopper (ii) ions in solution, the pale blue. reactions of copper(ii) ions in solution. If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths. Copper Blue Ions.
From www.youtube.com
Cation Test Copper(II) Ions YouTube Copper Blue Ions why is copper(ii) sulphate solution blue? like many transition metal complexes, copper (ii) sulfate pentahydrate is brightly colored; The simplest ion that copper forms in solution is the typical blue. If you add an excess of ammonia solution to hexaaquacopper (ii) ions in solution, the pale blue. Crystals of this beautiful substance are a. reactions of copper(ii). Copper Blue Ions.
From www.alamy.com
Diagram of the electrolysis of copper (II) chromate (VI) showing the movement of ions. Fully Copper Blue Ions copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Crystals of this beautiful substance are a. If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. The simplest ion that copper forms in solution is the typical blue. reactions of copper(ii) ions in solution. . Copper Blue Ions.
From www.researchgate.net
Copper ions coordination in the crystal structure of Cu[C 3 H 5 NH 2 ]N... Download Scientific Copper Blue Ions If you add an excess of ammonia solution to hexaaquacopper (ii) ions in solution, the pale blue. If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. The simplest ion that copper forms in solution is the typical blue. why is copper(ii) sulphate solution blue? Crystals of this beautiful substance are. Copper Blue Ions.
From woelen.homescience.net
Science made alive Chemistry/Solutions Copper Blue Ions Crystals of this beautiful substance are a. like many transition metal complexes, copper (ii) sulfate pentahydrate is brightly colored; copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. reactions of copper(ii) ions in solution. If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are.. Copper Blue Ions.
From www.youtube.com
When copper (II) ions are surrounded by chloride ions it turns from blue to green Chemistry Copper Blue Ions reactions of copper(ii) ions in solution. The simplest ion that copper forms in solution is the typical blue. like many transition metal complexes, copper (ii) sulfate pentahydrate is brightly colored; Crystals of this beautiful substance are a. If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. copper(ii) ion. Copper Blue Ions.
From www.reddit.com
Precipitate Colors of Metal Ions in Aqueous Ammonia and Sodium Hydroxide Solution Helpful A Copper Blue Ions copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. reactions of copper(ii) ions in solution. If you add an excess of ammonia solution to hexaaquacopper (ii) ions in solution, the pale blue. If white light (ordinary sunlight, for example) passes through copper(ii) sulphate solution, some wavelengths in the light are. reactions of. Copper Blue Ions.